Simple molecules -- symmetry and vibrations
Let us consider a simple
symmetric molecule:
C Cl4
(carbon tetrachloride)
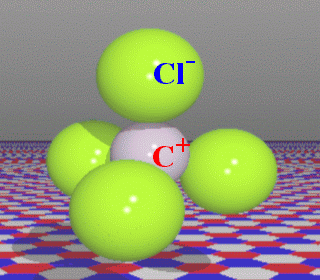 The
molecule consists of n=5 atoms, thus
the number of normal modes (excluding 3 translations and 3 rotations) will
be N=3n-6=9. Due to the high symmetry
(presence of mirror planes and three-fold axes) there will be doublets
and triplets of identical types of vibration (transformed into one another
by symmetry operations), with evidently indistinguishable frequencies.
This is called degeneracy. In our case, in the point of fact, only
4 out of 9 vibrations are different.
The
molecule consists of n=5 atoms, thus
the number of normal modes (excluding 3 translations and 3 rotations) will
be N=3n-6=9. Due to the high symmetry
(presence of mirror planes and three-fold axes) there will be doublets
and triplets of identical types of vibration (transformed into one another
by symmetry operations), with evidently indistinguishable frequencies.
This is called degeneracy. In our case, in the point of fact, only
4 out of 9 vibrations are different.
Let us take a look at these
different vibrations in motion...
 The
molecule consists of n=5 atoms, thus
the number of normal modes (excluding 3 translations and 3 rotations) will
be N=3n-6=9. Due to the high symmetry
(presence of mirror planes and three-fold axes) there will be doublets
and triplets of identical types of vibration (transformed into one another
by symmetry operations), with evidently indistinguishable frequencies.
This is called degeneracy. In our case, in the point of fact, only
4 out of 9 vibrations are different.
The
molecule consists of n=5 atoms, thus
the number of normal modes (excluding 3 translations and 3 rotations) will
be N=3n-6=9. Due to the high symmetry
(presence of mirror planes and three-fold axes) there will be doublets
and triplets of identical types of vibration (transformed into one another
by symmetry operations), with evidently indistinguishable frequencies.
This is called degeneracy. In our case, in the point of fact, only
4 out of 9 vibrations are different.