Climbing the first principle carbon hill
Climbing the first-principle Carbon Hill
Two classes of material-surface systems stand out in terms
their role of facillitating controlled experiments and thus
accelerating understanding in condensed matter physics:
- All-carbon materials: graphene, graphene systems, graphitics,
and their derivatives; We collective refer to these as Carbon Hill
since these materials also offer exciting possibilities for development
of new electronics and hence a possible alternative to silicon technology.
- Ultra-clean, well-characterized surfaces; in particular noble-metal surfaces
investigated in in ultra-high vacuum and probed by, for example, scanning-tunneling
microscopy (STM) or other surface-sensitive experimental techniques.
The importance of the first class systems has recently been highlighted by
a Nobel Prize in physics for the discovery of graphene; the importance of the second
class of system is the wealth of possibilities for STM probing and other precise
characterization techniques such well-defined surface systems permits.
Both classes of systems therefore defined exciting opportunities to develop
science through a careful experiment-theory calibration.
First-principle calculations have long played an indispensible role in
general materials physics but have had difficulties describing both the graphitics
(or more generally) supramolecular problems, and most physisorption problems.
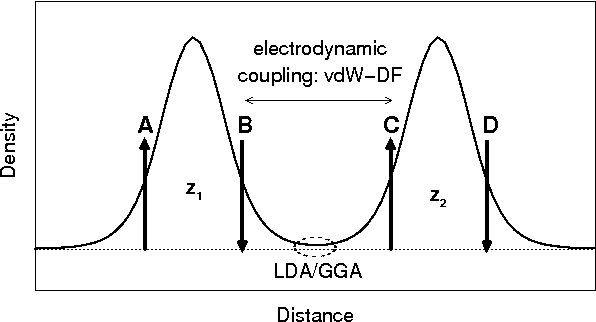
This is because such systems are sparse, i.e., the materials have important
regions with a sparse distribution of electrons.
Role of van der Waals forces on the Carbon Hill
Starting (mainly) from surface-physics theory, being electron density
functional theory (DFT) developers, and recognizing the imperative need for
a close experiment-theory comparison, we have at Chalmers and GU had a
long-standing involvement with the both classes of systems.
A recent parameter-free and predictive theoretical account of
both classes of materials-surface systems has come with our
Rutgers-Chalmers development of the van der Waals Density Functional
(vdW-DF) method for efficient sparse-matter DFT investigations.
The vdW-DF method is a framework for consistent nonempirical evaluations
of the exchange-correlation energy, going systematically beyond LDA and a
GGA, reaching a true nonlocality in the description of correlations.
The method is entirely based on the electron density variations
and takes no other input than nuclei charges and the parameters
that define constraint based LDA and GGA. The method has no
need of fitting a damping function and today, the use of
vdW-DF typically causes no descernable delay when compared
to, for example, a standard GGA functional. The
density fucntional method ahs been and is being developed
in a long-standing transatlantic collaboration that began
between the Rutgers research group of David C. Langreth
and the Chalmers research groups of Elsebeth Schröder,
of Bengt I. Lundqvist, and of Per Hyldgaard.
Sadly, David Langreth passed away in 2011. David is a
dearly missed freind and a mentor to many of us. David was
an outstanding condensed matter physicists who influenced
our thinking in many important ways. His many-body physics
formulation, with Bengt I Lundqvist and students, of the
plasmon framework that underpins vdW-DF enabled the
Rutgers-Chalmers collaboration to launch robust nonlocal
density functionals that is meeting with broad interests.
The emphasis on conservation laws and physics-based
constraints suggests a high degree of transferability
and the vdW-DF versions have been sucessfully tested for
many different types of materials systems.
Our network of core Rutgers-Chalmers vdW-DF stakeholders
also includes the research groups of Timo Thonhauser (Wake Forest),
Valentino Cooper (Oak Ridge), and Kuyho Lee (Molecular Foundry,
Berkeley Labs) who are also key Rutgers contributors.
The group of core Rutgers-Chalmers vdW-DF stakeholders welcome
questions on the method and it use. We are committed to
continue to extend the method usefulness, efficientcy, and
leverage, for example, by connecting vdW-DF calculations to
wider materials and system modeling. The group
of vdW-DF stakeholders are also continuing the method
development work through many-body physics analysis and
strictly within a constraint-based plasmon framework
that makes the vdW-DF method a close relative of LDA and GGA.
We separately maintain an
overview of Chalmers-based
vdW-DF contributions since the range of vdW-DF applications is not
limited to carbon materials (or surfaces or molecular adsorption)
but extends to the very broad class of spare matter, materials with
important voids in the electron distributions.
The vdW-DF method not only provides a first parameter-free quantum-physical
account of the Carbon Hill and molecular adsorbate on metal surface problems.
Graphene and Graphitics also holds a special role in driving the development and
implementations of vdW-DF. In fact, it was the question "what drives the assembly of
individual (graphene) sheets into graphite?" which got us started on doing a
full implemention of the vdW-DF method in regular state-of-the-art DFT
calculation schemes (and later in the codes themselves) early in the new millenium.
Gallery of first-principle carbon hill results
Reflecting also on the key importance of both classes of exciting materials-surface
systems, we summarize below our key First-Principle Carbon Hill results while we refer
to a separate presentation of a corresponding
First-Principle
insight on noble-metal and other surface webpage. At both of these websites we collect
contributions that have an address at the above-mentioned (vdW-DF developing) Chalmers
research groups.
Invited Reviews on the vdW-DF method and on graphene and graphitics systems
|
Density-functional bridge between surfaces and interfaces,
(Lundqvist et al) Surface Science 493, 253 (2001).
(preprint).
Contains, among other things, our first (2001) presentation of vdW-DF study of binding of graphene.
|
|
|
 |
Bridging between micro- and macroscales of
materials by mesoscopic models,
(Lundqvist et al) Computational Materials Science 24, 1 (2002).
(preprint).
|
|
|
 |
Van der Waals Density Functional Theory with Applications
(Langreth, Dion, Rydberg, Schröder, Hyldgaard, Lundqvist)
International Journal of Quantum Chemistry 101, 599 (2005).
preprint
|
|
|
 |
A density functional for sparse matter,
(Langreth et al) Journal of Physics: Condensed Matter 21, 084203 (2009).
Copyright (2009) by IoP.
|
|
|
 |
van der Waals density functionals build upon the electron gas tradition: Facing the
challenge of compeeting interactions,
(Berland, Arter, Cooper, Lee, Lundqvist, Schröder, Thonhauser, and Hyldgaard)
J. Chem. Phys. 140, 18A539 (2014),
For special JCP issue on
``Methods in Density Functional Theory.''
An invited contributions on the
van der Waals density functional (vdW-DF) method.
|
|
|
 |
van der Waals forces in density functional theory:
A review of the vdW-DF method,
(Berland, Cooper, Lee, Schröder, Thonhauser, Hyldgaard,
and Lundqvist)
Reports of Progress in Physics 78, 066501 (2015).
An invited review on the long-term research investments and progress
that the van der Waals
density functional (vdW-DF) method represents.
|
|
|
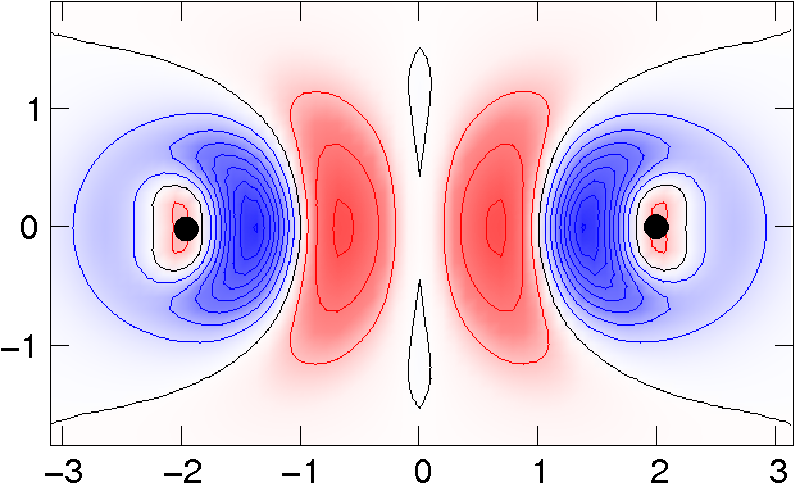
Density functional theory: Details of the vdW-DF method and more
 |
Van der Waals Density Functional for Layered Structures,
(Rydberg, Dion, Jacobson, Schröder, Hyldgaard, Simak, Langreth,
Lundqvist) Physical Review Letter 91, 126402 (2003).
Copyright (2003) by the American Physical
Society.
|
|
|
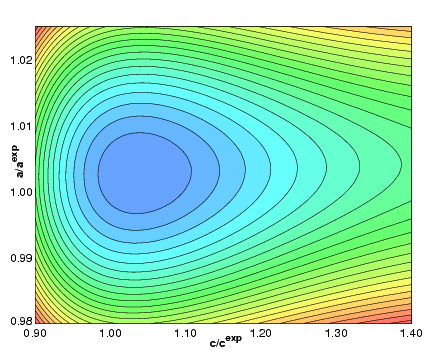
 |
Van der Waals Density Functional for
General Geometries,
(Dion, Rydberg, Schröder, Lundqvist, and Langreth) Physical Review Letters 92, 246401 (2004).
Copyright (2004) by the American Physical Society.
Erratum
|
|
|
 |
Van der
Waals density functional: Self-consistent potential and the nature of the van der Waals bond,
(Thonhauser, Cooper, Li, Puzder, Hyldgaard, and Langreth) Physical Review B 76, 125112 (2007).
Copyright (2007) by the American Physical Society.
|
|
|
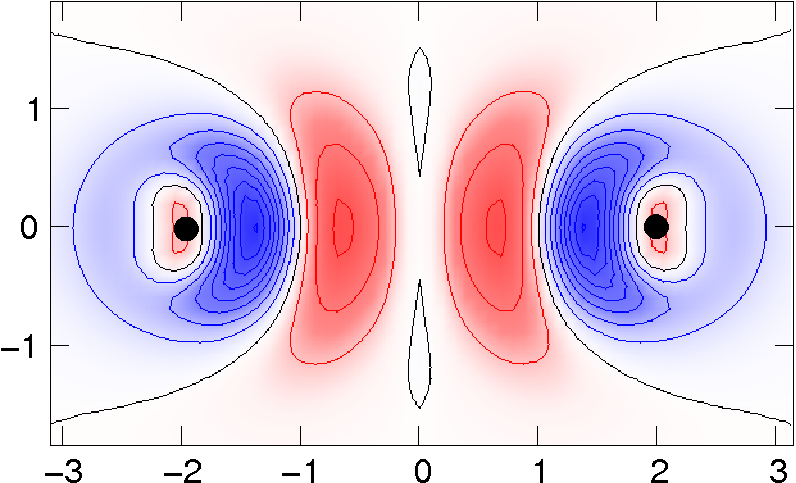
 |
Higher-accuracy van der Waals density functional,
(Lee, Murray, Kong, Lundqvist, and Langreth) Physical Review B 82, 081101(R) (2010).
|
|
|
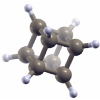 |
Structure and binding in crystals of cage-like molecules:
hexamine and platonic hydrocarbons,
(Berland and Hyldgaard) Journal of Chemical Physics 132, 134705 (2010).
cond-mat/1010.1487.
|
|
|
 |
An analysis of van der Waals density functional components: Binding
and corrugation of benzene and C60 on boron nitride and graphene,
(Berland and Hyldgaard) Physical Review B 87, 205421 (in 2013).
Copyright (2013) by American Physical Society.
|
|
|
 |
Exchange functional that tests the robustness of the plasmon descripion of the van der Waals density
functional,
(Berland and Hyldgaard)
Physical Review B 89, 035412 (2014).
Copyright (2014) by the American Physical
Society.
|
|
|
 |
van der Waals density functionals based on the electron-gas
tradition: facing the challenge of
compeeting interactions,
(Berland, Arter, Cooper, Lee, Lundqvist, Schröder, Thonhauser, and
Hyldgaard)
Journal of Chemical Physics 140, 18A539 (2014);
invited contribution to special JCP issue on ``Methods in Density
Functional Theory.''
cond-mat/1403.0797.
|
|
|
 |
Spin signature of nonlocal-correlation binding in metal organic
frameworks,
(Thonhauser, Zuluaga, Arter, Berland, Schröder, and hyldgaard)
Physical Review Letters 115, 136402 (2015).
Copyright (2015) by the American Physical
Society.
|
|
|
Density functional theory: vdW-DF implementation, benchmarking and
computational strategy papers
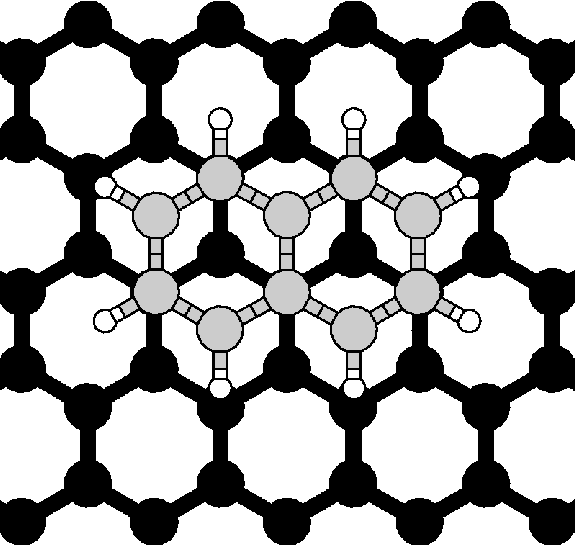 |
Application
of van der Waals Density Functional to an Extended System:
Adsorption of Benzene and Naphthalene on Graphite,
(Chakarova-Käck, Schröder, Lundqvist, and Langreth)
Physical Review Letters 96 (2006) 146107.
Copyright (2006) by the American Physical Society.
|
|
|
|
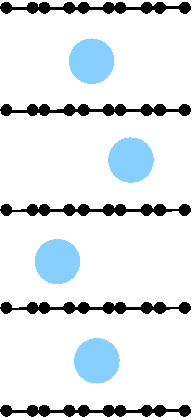
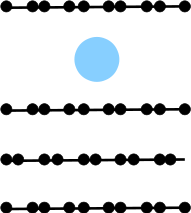
Potassium intercalation in graphite: A van der Waals
density-functional study
(Ziambaras, Kleis, Schröder, and Hyldgaard) Physical Review B 76, 155425 (2007).
Copyright (2007) by the American Physical Society.
|
|
|
 |
Nature and strength of bonding in a crystal of semiconducting nanotubes: van der Waals density
functional calculations and analytical results
(Kleis, Hyldgaard, and Schröder) Physical Review B 77, 205422 (2008).
Copyright (2008) by the American Physical Society.
The article was selected to simultaneously appear in the
June 2, 2008 issue of the AIP/APS Virtual Journal of
Nanoscale Science & Technology.
|
|
|
 |
van der Waals density functional calculations of binding
in molecular crystals,
(Berland, Borck, and Hyldgaard) Computational Physics Communications
182, 1800 (2011).
cond-mat/1007.3305.
|
|
|
 |
Vanadium pentoxide (V2O5):
a van der Waals density functional study,
(Londero and Schröder)
Computer Physics Communications 182, 1805 (2011).
|
|
|
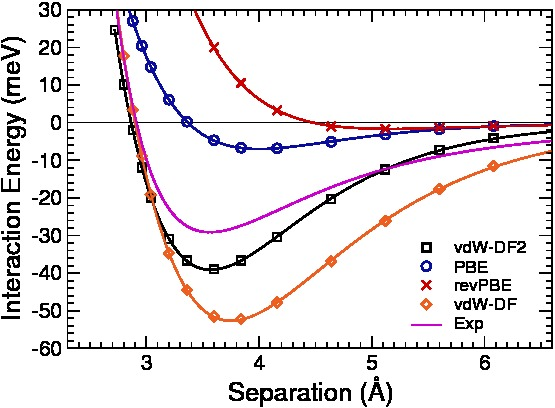 |
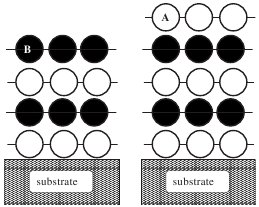
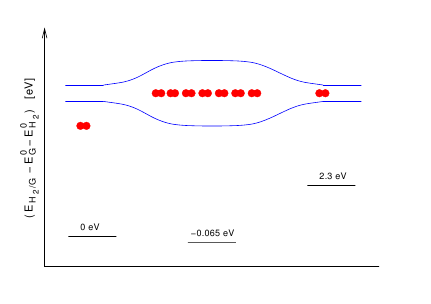
Evaluation of a density functional with account of van der
Waals forces using experimental
data of H2 physisorption on Cu(111)
(Lee, Kelkkanen, Berland, Andersson, Langreth,
Schröder, Lundqvist, and Hyldgaard)
Physical Review B 84, 193408 (2011).
Copyright (2011) by American Physical
Society.
|
|
|
 |
Physisorption of nucleobases on graphene: a comparative van der Waals
study,
(Le, Kara, Hyldgaard, E. Schröder, and T.S. Rahman)
Journal of Physics:Condensed Matter 24, 424210 (2012).
Copyright (2012) by IoP.
|
|
|
 |
Benchmarking van der Waals density functionals with experimental
data: potential energy curves for H2 molecules on Cu(111), (100), and
(110)
surfaces
(Lee, Berland, Yoon, Andersson, Schröder, Hyldgaard, and Lundqvist)
Journal of Physics:Condensed Matter 24, 424213 (2012).
Copyright (2012) by IoP.
|
|
|
 |
Harris-type van der Waals density functional scheme,
(Berland, Londero, Schröder, and Hyldgaard)
Physical Review B 88, 045431 (2011).
Copyright (2013) by American Physical
Society.
|
|
|
 |
Structural and excited-state properties of oligoacene crystals from
first principles,
(Rangel, Berland, Sharifzadeh, Altvater, Lee, Hyldgaard, Kronik, and Neaton)
Physical Review B 93, 115206 (2016).
Copyright (2016) by American Physical
Society.
|
|
|
Within and beyond canonical-ensemble ground-state density
functional theory: thermodynamics and
nonequilibrium tunneling
 |
Ab initio thermodynamics of deposition growth: surface terminations of CVD
titanium carbide and nitride,
(Rohrer and Hyldgaard) Physical Review B 82, 045415 (2010).
Copyright (2010) by American Physical Society.
|
|
|
 |
Graphene Nanogab for Gate Tunable Quantum Coherent Single Molecule Electronics
,
(Bergvall, Berland, Hyldgaard, Kubatkin, and Löfwander)
Physical Review B 84, 155451 (2011).
cond-mat/1108.4571.
|
|
|
 |
Nonequilibrium thermodynamics of interacting tunneling transport:
variational grand potential, universal density functional description,
and nature of forces,
(Hyldgaard) Journal of Physics:Condensed Matter 24, 424219
(2012).
Copyright (2012) by IoP.
cond-mat/1108.4536.
|
|
|
Graphene and graphene-related systems
 |
Van der Waals Density Functional for Layered Structures,
(Rydberg, Dion, Jacobson, Schröder, Hyldgaard, Simak, Langreth,
Lundqvist) Physical Review Letter 91, 126402 (2003).
Copyright (2003) by the American Physical
Society.
|
|
|
 |
Hydrogen dynamics in magnesium and graphite,
Computational Materials Science 24, 273 (2002).
(preprint).
|
|
|
 |
Hard numbers on soft matter,
(Rydberg, Jacobson, Hyldgaard, Simak, Lundqvist,
and Langreth) Surface Science 532-535, 606-610 (2003).
(preprint).
|
|
|


|
Potassium intercalation in graphite: A van der Waals
density-functional study
(Ziambaras, Kleis, Schröder, and Hyldgaard) Physical Review B 76, 155425 (2007).
Copyright (2007) by the American Physical Society.
|
|
|
 |
A van der Waals density functional study of adenine on graphite: Single
molecular adsorption and overlayer binding
(Berland, Chakarova-Käck, Cooper, Langreth, and Schröder)
Journal of Physics: Condensed Matter 23, 135001 (2011).
cond-mat/1009.5793.
|
|
|
 |
Role
of van der Waals bonding in the layered oxide V2O5: First-principles
density-functional calculations,
(Londero and Schröder) Physical Review B 82, 054116 (2010).
Copyright (2010) by American Physical Society.
|
|
|
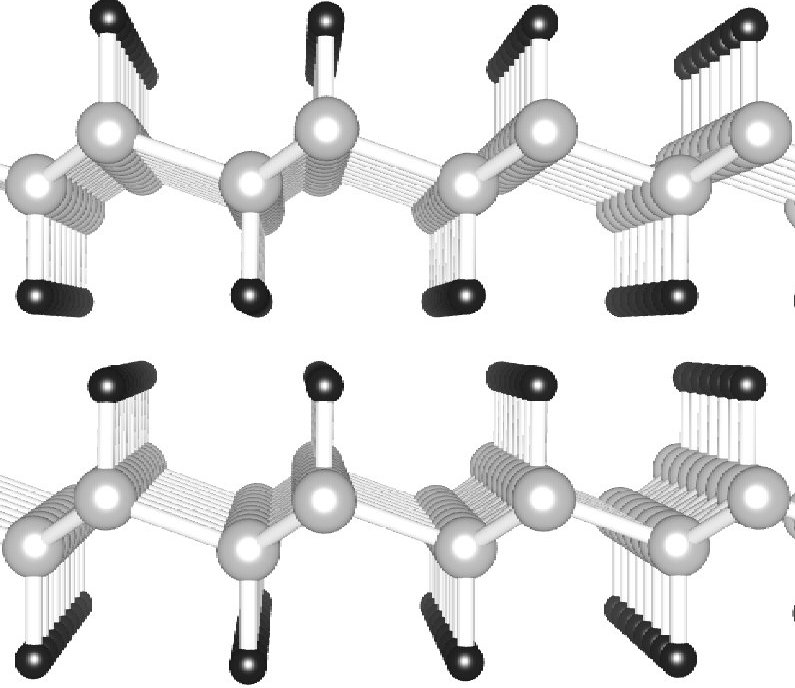 |
Stacking and band structure of van der Waals bonded graphane multilayers
,
(Rohrer and Hyldgaard) Physical Review B 83, 165423 (2011).
Copyright (2011) by the American Physical Society.
|
|
|
 |
An analysis of van der Waals density functional components: Binding
and corrugation of benzene and C60 on boron nitride and graphene,
(Berland and Hyldgaard)
Physical Review B , 205421 (2013).
Copyright (2013) by the American Physical Society.
|
|
|
 |
Microscopic origin of thermal conductivity reduction in disordered
van der Waals solids.
(Erhart, Hyldgaard, and Lindroth) Chemistry of Materials, 27,
5511 (2015).
|
|
|
Nanotubes, polymers, and biopolymer (DNA) systems
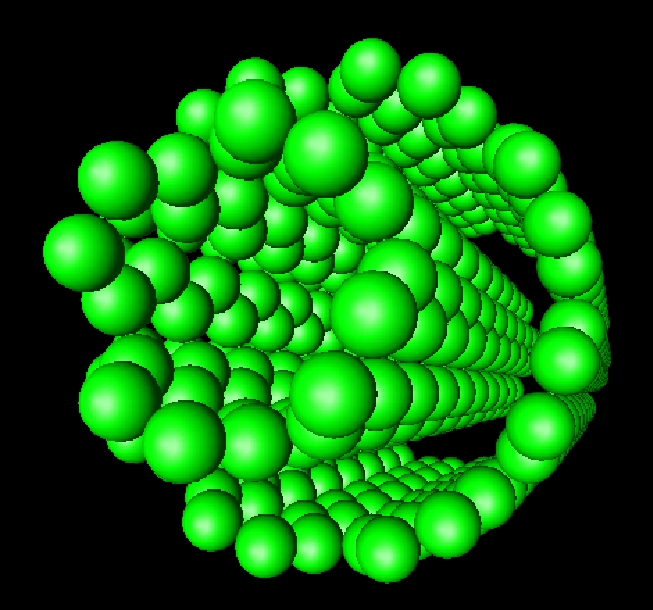 |
The
van der Waals Interactions of Concentric Nanotubes,
(Schröder and Hyldgaard) Surface Science 532, 880-885 (2003).
|
|
|
 |
van der Waals Interactions of Parallel and Concentric
Nanotubes,
(Schröder and Hyldgaard) Materials Science and Engineering C 23, 721 (2003).
cond-mat/0307017.
|
|
|
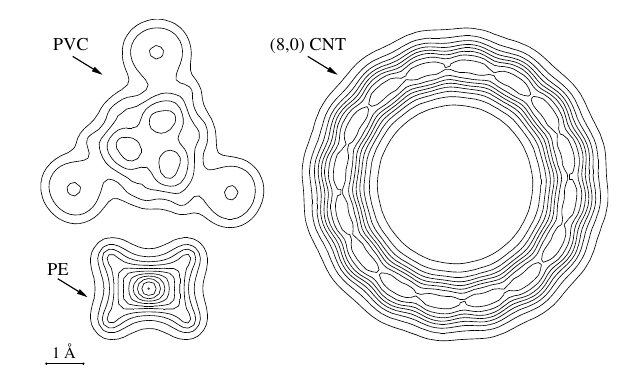 |
Van der Waals interaction of parallel polymers and nanotubes
(Kleis, Hyldgaard, and Schröder) Computational Materials Science 33 (2005) 192.
cond-mat/0407107.
|
|
|
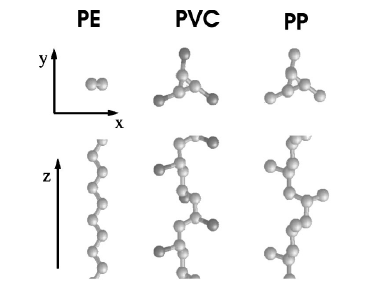 |
Van
der Waals interaction of simple, parallel polymers,
(Kleis and Schröder) Journal of Chemical Physics 122 (2005) 164902.
|
|
|
 |
Towards
a working density-functional theory for polymers: First-principles determination of
the polyethylene crystal structure,
(Kleis, Lundqvist, Langreth, Schröder)
Physical Review B 76 (2007) 100201(R).
Copyright (2007) by the American Physical Society.
|
|
|
 |
Nature and strength of bonding in a crystal of semiconducting nanotubes: van der Waals density
functional calculations and analytical results
(Kleis, Hyldgaard, and Schröder) Physical Review B 77, 205422 (2008).
Copyright (2008) by the American Physical Society.
The article was selected to simultaneously appear in the
June 2, 2008 issue of the AIP/APS Virtual Journal of
Nanoscale Science & Technology.
|
|
|
 |
Stacking Interactions and the Twist of DNA
(Cooper, Thonhauser, Puzder, Schröder,
Lundqvist, and Langreth) Journal of the American Chemical Society 130 (2008) 1304.
|
|
|
 |
A van der Waals density functional mapping of attraction in
DNA dimers,
(Londero, Hyldgaard, and Schröder) to be submitted (in 2013).
cond-mat/1304.1936.
|
|
|
Molecular dimers, crystals, and Buckyball systems
 |
van der Waals interactions of the benzene dimer:
towards treatment of polycyclic aromatic hydrocarbon dimers
(Chakarova-Käck and Schröder) Materials Science and Engineering C 25 (2005) 787.
cond-mat/0406549.
|
|
|
 |
van der Waals interactions of polycyclic
aromatic hydrocarbon dimers,
(Chakarova-Käck and Schröder) Journal of Chemical Physics 122 (2005) 054102.
|
|
|
 |
Binding of polycyclic aromatic hydrocarbons and graphene
dimers in density functional theory,
(Chakarova-Käck, Vojvodic, Kleis, Hyldgaard, and
Schröder) New Journal of Physics 12, 013017 (2010).
|
|
|
 |
van der Waals density functional calculations of binding
in molecular crystals,
(Berland, Borck, and Hyldgaard) Computational Physics Communications
182, 1800 (2011).
cond-mat/1007.3305.
|
|
|
 |
Harris-type van der Waals density functional scheme,
(Berland, Londero, Schröder, and Hyldgaard)
Physical Review B 88, 045431 (2011).
Copyright (2013) by American Physical
Society.
|
|
|
Surface-bonding of molecules: benzene, acenes, PAHs and their derivatives
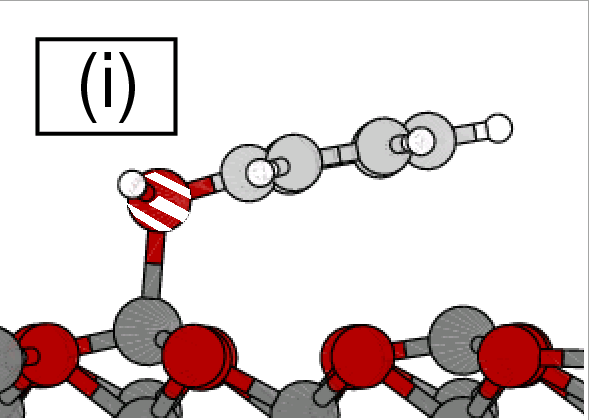 |
Adsorption of phenol on graphite(0001) and
α-Al2O3(0001):
Nature of van der Waals bonds from first-principles calculations
(Chakarova-Käck, Borck, Schröder, Lundqvist) Physical Review B 74 (2006) 155402.
Copyright (2006) by the American Physical Society.
|
|
|
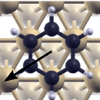 |
Rings sliding on a honeycomb network: Adsorption contours, interactions, and assembly of benzene on
Cu(111),
(Berland, Einstein, and Hyldgaard) Physical Review B 80, 155431 (2009).
Copyright (2009) by the American Physical Society.
|
|
|
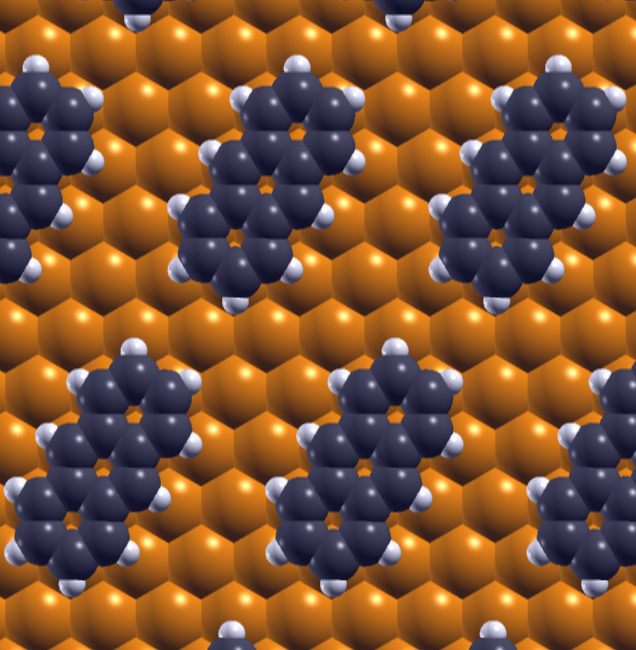 |
Effective Elastic Properties of a Molecular Monolayer at a Metal Surface ,
(Sun, Kim, Le, Borck, Berland, Kim, Lu, Luo, Cheng, Einstein, Rahman, Hyldgaard, Bartels)
Physical Review B 82, 201410(R) (2010).
Copyright (2010) by the American Physical Society.
|
|
|
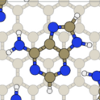 |
A van der Waals density functional study of adenine on graphite:
Single
molecular adsorption and overlayer binding
,
(Berland, Chakarova-Käck, Cooper, Langreth, and Schröder)
Journal of Physics:Condensed Matter 23, 135001 (2011).
cond-mat/1009.5793.
|
|
|
 |
Do two-dimensional "Noble Gas Atoms" Produce Molecular Honeycombs at
a Metal Surface,
(Wyrick, Kim, Sun, Cheng, Lu, Zhu,
Berland, Kim, Rotenberg,
M. Luo, Hyldgaard, Einstein, Bartels)
Nano Letters 11, 2944 (2011).
|
|
|
NanoIC innovation program: Graphene, and carbon-system, abchoring, formation and binding
November 20, 2011 by
Per Hyldgaard